Tumor-associated macrophages (TAMs) are among the most abundant immune cells within the tumor microenvironment (TME) and act as key regulators of various tumor-associated immune responses. In a previous discussion, we focused on the interactions between TAMs and other TME components, as well as the different subtypes of TAMs. In this article, we delve into the direct connections between TAMs and cancer cells, metabolic pathways within TAMs that can be targeted for therapeutic purposes, and the current state of macrophage-based cell therapy development.
▣ Myeloid Checkpoints and Regulators
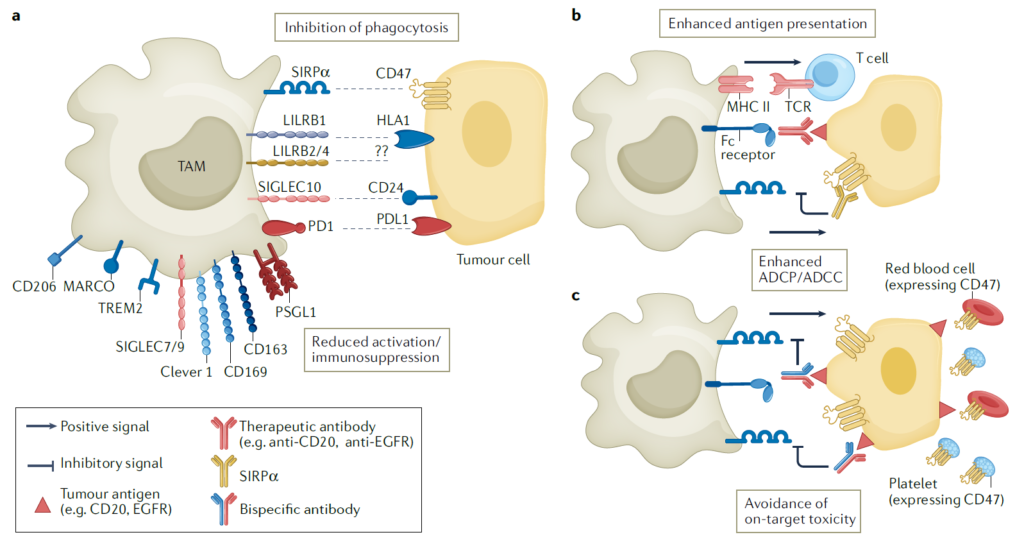
The function of myeloid monocytes is regulated by direct regulators, which can be classified as myeloid checkpoints or scavenger receptors. These checkpoints and receptors establish an immunosuppressive environment while facilitating the migration of TAMs to tumor tissue. However, tumor cells can exploit these mechanisms to evade immune surveillance.
1) SIRPa and CD47
CD47 is a protein widely expressed on normal cells, functioning as a “don’t eat me” signal to avoid phagocytosis by macrophages and neutrophils when it binds to SIRPa. Cancer cells also exploit this mechanism to evade anti-tumor immune responses by expressing CD47, so blocking the CD47-SIRPa axis can promote the elimination of cancer cells and activation of T cells. Thus, targeting CD47 has shown enhanced efficacy when combined with T-cell immune checkpoint inhibitors. However, because CD47 is also broadly expressed on normal cells such as red blood cells and platelets, blocking it could lead to side effects like anemia and thrombocytopenia. To mitigate these effects, alternative strategies have been developed, such as targeting SIRPa directly, using IgG4 antibodies with lower affinity for NK cells, or employing bispecific antibodies that target both CD47 and tumor-specific antigens to improve cancer cell selectivity.
2) SIGLEC Family
The SIGLEC family consists of membrane proteins that bind to sialic acid. They contain intracellular tyrosine-based inhibitory motifs (ITIMs), which deliver immunosuppressive signals to regulate immune cell functions. In cases where SIGLEC1 is expressed on TAMs and tumors, poor prognosis has been observed. However, when SIGLEC1-expressing TAMs are depleted, tumor size and metastasis are reduced. Proteins such as SIGLEC7 and 9, other members of this family, have also shown similar trends.
3) LILRB Family
Cancer cells also use interactions between major histocompatibility complex class 1 (MHC I) and LILRB proteins to evade phagocytosis. LILRB1, for instance, is expressed widely in immune cells, including TAMs. Like SIGLEC proteins, LILRB contains an ITIM motif, contributing to immunosuppressive signaling. Blocking LILRB2 in LILRB2+ macrophages enhances inflammatory responses and phagocytic function. MK-4830, an antibody developed to block LILRB2, has completed a Phase I trial for terminal-stage solid cancers. The results showed increased secretion of inflammatory cytokines, such as GM-CSF and TNF, as well as enhanced cytotoxic T-cell activity. Another protein in the same family, LILRB4, is known to be highly expressed in TAMs infiltrating the tumor, strongly suppressing tumor-related immune responses. Blocking LILRB4 can also re-activate tumor-infiltrating T cells or induce TAMs to differentiate into an activated phenotype.
4) Scavenger Receptors
Scavenger receptors are frequently observed in TAMs and act as inflammation switches, showing potential as therapeutic targets. Among these receptors, CD163+ macrophages have been clinically associated with tumor growth, with significant findings in pancreatic cancer and melanoma. Depleting CD163+ macrophages could reduce tumors resistant to anti-PD1 therapy, potentially enhancing TME remodeling and the infiltration of T cells and inflammatory monocytes.
CD206 is a receptor that binds to components of pathogens. When it binds to ligands or target antibodies, it promotes an immunosuppressive phenotype by increasing IL-10 production. RP-182, a peptide drug targeting CD206, binds to CD206 and alters its structure. When treated with RP-182, CD206+ macrophages were reduced or transformed into M1-like phenotypes with anti-tumor characteristics, inhibiting tumor growth.
Another scavenger receptor, MARCO, is also highly expressed in TAMs. Using anti-MARCO antibodies has been found to induce anti-tumor immune responses and reprogram TAMs toward an inflammatory phenotype. Unlike other targets, anti-MARCO antibodies enhance the cytotoxic function of NK cells rather than CD8+ T cells.
Finally, the Clever1 receptor binds to lipoproteins or carbohydrates to mediate their internalization, transferring the absorbed molecules to endosomes, and regulates immunosuppressive signals in macrophages and T cells. When Clever1 is blocked, TAMs switch from a suppressive to an inflammatory phenotype, resulting in T cell activation. FP-1305, an anti-Clever1 antibody developed for this purpose, was modified to regulate Fcγ-mediated cytotoxicity and complement-dependent functions, reducing side effects due to the expression of Clever1 on lymphatic vessels.
5) TREM2 (Triggering Receptor Expressed on Myeloid Cells 2)
TREM2 is expressed in macrophages of specific tissues and has been observed to be overexpressed in TAMs within tumors. Targeting and inhibiting TREM2+ macrophages has shown the potential to suppress tumor growth and improve responsiveness to anti-PD1 therapies. A monoclonal antibody targeting TREM2, PY314, is currently in Phase I clinical trials for end-stage solid cancers.
6) PSGL1
PSGL1 is expressed on hematopoietic-derived cells and is induced via M2 differentiation signals, showing high expression in TAMs. Blocking PSGL1 with an antibody reportedly reprograms M2 macrophages into M1 macrophages and restores immune responses suppressed within the TME, aiding in anti-cancer effects.
▣ Targeting Macrophage Metabolic Pathways
Macrophages, which differentiate into various subtypes, undergo detailed changes in amino acid, lipid, and iron metabolism to switch their function or phenotype. By targeting these metabolic pathways, it is possible to modulate the responsiveness of macrophages within the tumor environment.
1) Epigenetic Targets
Regulating the epigenetic stages of macrophages can alter their function by inhibiting the activity of HDACs (Histone deacetylases). Inhibitors of HDACs, such as TMA195 and tefinostat, have shown promising results in clinical trials for end-stage hematologic cancers.
2) Glycolysis
Lactate, a by-product of the glycolysis pathway in cancer cells, promotes differentiation of macrophages into an immunosuppressive M2-like phenotype. Thus, blocking glycolysis is a potential strategy to reprogram TAMs. However, glycolysis is crucial for the anti-tumor functions of macrophages, leading to some debate over blocking glycolysis directly. Metformin, a glycolysis inhibitor, has already been studied in the clinical setting for certain cancer types. Metformin has been reported to restructure the TME, reducing TAM density and promoting apoptosis.
3) Glutamine Metabolism
M2-like immunosuppressive TAMs tend to increase glutamine consumption. Reducing this metabolism through small molecules or inhibiting synthetic enzymes can promote macrophage reprogramming, slowing tumor growth.
4) Tryptophan Metabolism
The increased expression of IDO-1 in TAMs boosts the consumption of tryptophan, an essential amino acid for T cell activation. Tryptophan depletion by TAMs impairs T cell activation and increases immunosuppressive regulatory T cells. Clinical trials with IDO inhibitors are underway to counter this, and findings suggest that substituting IDO1 with TDO or IDO2 instead of full IDO inhibition may yield more positive outcomes.
5) Lipid Metabolism
Among metabolic disorders in TAMs, lipid dysregulation plays a significant role. Lipid metabolism dysregulation in TAMs activates immunosuppressive signaling through LXR, an oxysterol receptor that influences transcription factors. Based on this mechanism, strategies targeting LXR have been developed, and synthetic LXR agonists have enhanced anti-inflammatory effects. Prostaglandins, a type of lipid, serve as inflammatory mediators and regulate mechanisms of tumor evasion. In particular, PGE2 produced by tumors inhibits NK cell activation and inflammatory responses in myeloid cells, shifting their phenotype toward immunosuppression. To counter this, using COX2 inhibitors (which target prostaglandin synthesis) or EP1/EP2 antagonists (which target PGE2 receptors) can re-activate anti-cancer factors and enhance the efficacy of immune checkpoint inhibitors.
▣ Macrophage Therapies
TAMs are continuously replenished and maintained by circulating monocytes. Unlike CAR-T cells, which are effective in treating blood cancers but struggle to infiltrate and survive in solid tumors, macrophage-based cell therapies offer new possibilities for treating solid tumors due to their ability to penetrate the tumor. Macrophages can act like a Trojan horse, delivering cytokines or nanoparticles into the TME, or they can be genetically modified to act as phagocytes with specific receptors. This approach has been experimentally validated: monocytes loaded with drug-carrying nanoparticles, when injected intravenously, delivered the drugs more effectively to the tumor site than nanoparticles alone.
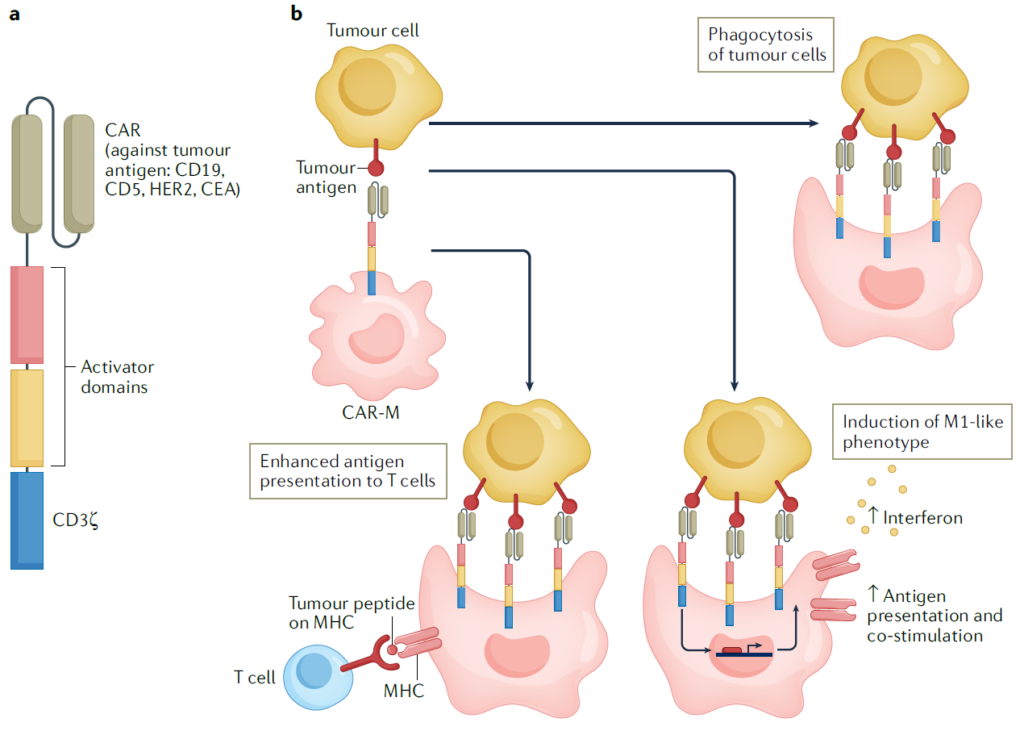
However, challenges persist in macrophage-based cell therapies due to the difficulty in reprogramming human macrophages. Efforts to overcome this obstacle are ongoing, with multiple technological platforms aiming to develop effective reprogramming strategies.
Moreover, CAR-M cells, engineered to express receptors that recognize tumor-associated factors, have been developed to target both hematologic cancers and solid tumors. CAR-M cells reliably exhibit phagocytic activity and maintain M1 phenotypic characteristics, showing efficacy against both primary and metastatic tumors. Clinical trials with CAR-M cells are currently underway, with plans to apply them across various cancer types.
Historically, anti-cancer therapies have primarily focused on using T cells or NK cells, which directly interact with tumor cells. However, these strategies alone have proven insufficient for treating solid tumors. Recently, therapeutic approaches have emerged that target macrophages, which are abundantly present in the TME. A particularly attractive quality of macrophages is their phenotypic diversity and plasticity, enabling them to transition between various phenotypes. While this characteristic is exciting from a drug development perspective, it also presents certain challenges. Although macrophage-targeted or macrophage-based cell therapies are not yet fully developed due to unclear mechanisms, recent advancements in scientific technology are gradually elucidating aspects of macrophage metabolism and differentiation. Leveraging these insights may help overcome the limitations of traditional anti-cancer therapies, potentially leading to the development of groundbreaking cancer treatments.
Reference : Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022 Nov;21(11):799-820. doi: 10.1038/s41573-022-00520-5.
