In the previous article, we provided a general overview of extracellular vesicles (EVs) along with their biogenesis and subtypes. In today’s article, we will focus more specifically on exosomes, exploring the rationale and research cases that support their potential as therapeutic approaches.
◎ Exosomes in immune system
The role of exosomes in the immune system has been continuously studied, yet no significant reports have been made regarding immune toxicity reactions caused by exosomes. For instance, mice that were repeatedly administered low doses of human- or mouse-derived exosomes did not exhibit severe immune responses. Similarly, no immune toxicity related to extracellular vesicles (EVs) has been observed in whole blood or plasma transfusions.
Although toxicity has not been prominently reported, various experiments have demonstrated that exosomes play specific roles in both innate and adaptive immunity. The immune regulatory functions induced by exosomes include antigen delivery and presentation, DNA-induced cGAS-STING signaling, gene expression regulation through microRNA (miRNA) transfer, and the activation of specific signaling pathways via exosomal surface proteins.
Exosomes secreted by antigen-presenting cells (APCs) carry MHC II molecules with antigenic peptides and costimulatory signals, allowing them to directly present antigens to T cells and induce their activation. Research has shown that a single subcutaneous injection of APC-derived exosomes in mice led to complete tumor regression or inhibition of tumor growth. Additionally, exosomes contribute to indirect antigen presentation between APCs, playing a role in the activation of naïve T and B cells.
Beyond simple antigen presentation and T/B cell activation, exosomes also impact adaptive immunity by actively promoting the production and secretion of IFN-α, IFN-γ, and TNF-α in response to bacterial infections.
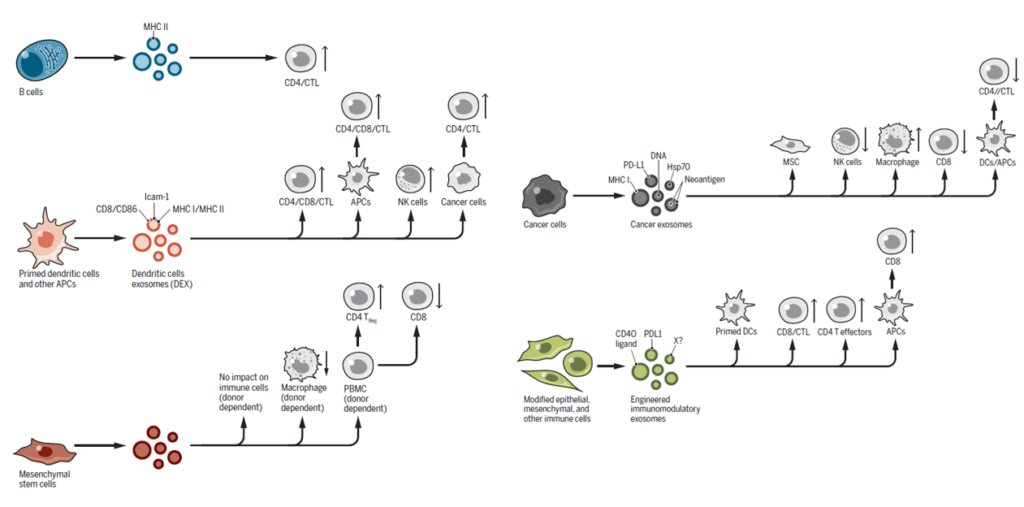
The nucleic acids contained within exosomes, such as DNA and microRNA (miRNA), also perform specific functions in the immune system. During bacterial infections, bacterial DNA can be carried within exosomes and delivered to recipient cells, where it stimulates the cGAS-STING signaling pathway, thereby activating the innate immune response. In tumor environments, tumor-derived exosomes can promote dendritic cell activation and cGAS-STING signaling, leading to tumor growth suppression. Conversely, the spread of tumor-derived exosomal DNA may accelerate cancer progression, and when engulfed by neutrophils, it can contribute to paraneoplastic conditions, such as thrombosis.
Exosomal miRNA influences the signaling pathways and gene expression of recipient cells. For instance, miR-212-3p suppresses RFXAP, a transcription factor for MHC II in dendritic cells, thereby facilitating tumor immune evasion.
Exosomes are also involved in the expression of immune checkpoint molecules. Tumor-derived exosomes suppress dendritic cell function via PD-L1–dependent mechanisms, leading to T cell exhaustion in lymph nodes. Additionally, exosomes can deliver FasL, inducing T cell apoptosis, which weakens the adaptive immune response and maintains the immunosuppressive state within the tumor microenvironment (TME). In contrast, mast cell–derived exosomes express MHC II, CD86, LFA-1, and ICAM-1, which enhance T and B cell proliferation.
These findings suggest that the functions of exosomes are determined by their cellular origin. Moreover, exosomes exhibit dual roles in the immune system, functioning as both activators and suppressors of immune responses. Regardless of the disease type—whether bacterial or viral infections, cancer, or other immune-related disorders—exosomes and their cargo derived from various cells play diverse and complex roles in immune regulation.
◎ Exosomes in various disease
Based on the role of exosomes in the immune system, we will categorize their pathological functions in different diseases.
1) Metabolic and Cardiovascular diseases
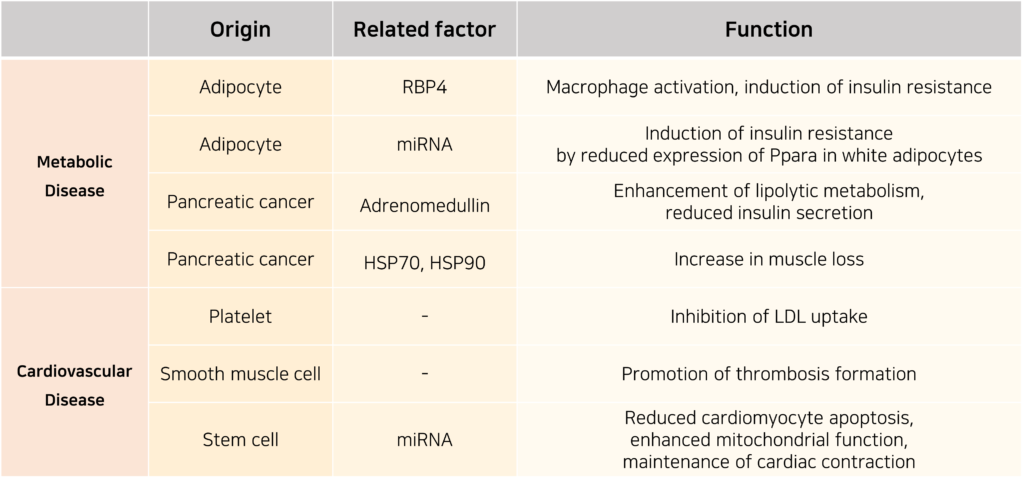
Exosomes are also implicated in the onset of metabolic disorders and cardiovascular health. They facilitate intercellular communication by mediating the exchange of metabolic molecules and the transfer of miRNA through exosomes. Through these mechanisms, exosomes contribute to both disease progression and homeostasis maintenance.
2) Neurodegenerative disease
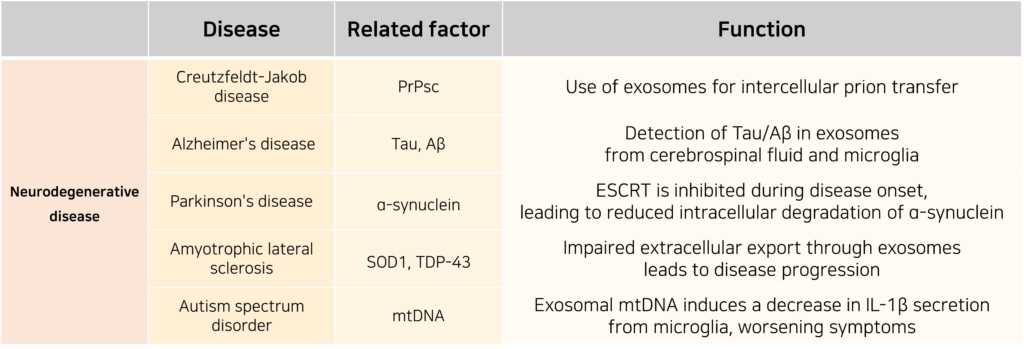
The similarities between exosome biogenesis mechanisms and the regulation of neuronal vesicle secretion suggest a significant link between exosomes and neurodegenerative diseases. Exosomes influence the progression of these diseases by either inhibiting or promoting the formation and aggregation of abnormal proteins in the brain.
In some cases, exosomes exhibit contradictory pathological roles. While they can help remove neurotoxic proteins from cells, thereby preventing aggregate formation, they can also facilitate the spread of these pathogenic proteins to other cells.
Exosomes are particularly noteworthy in neurodegenerative diseases not only because of their potential as diagnostic biomarkers but also due to their ability to cross the blood-brain barrier (BBB), making them promising candidates for therapeutic applications.
3) Cancer
Compared to other diseases, research on exosomes in cancer has progressed rapidly, revealing significant correlations between exosomes and cancer characteristics. Exosomes are involved in tumor formation, metastasis, paraneoplastic syndromes, and therapy resistance. However, their functions can be highly complex, depending on the cancer type, the patient’s genetic factors, and the stage of cancer progression.
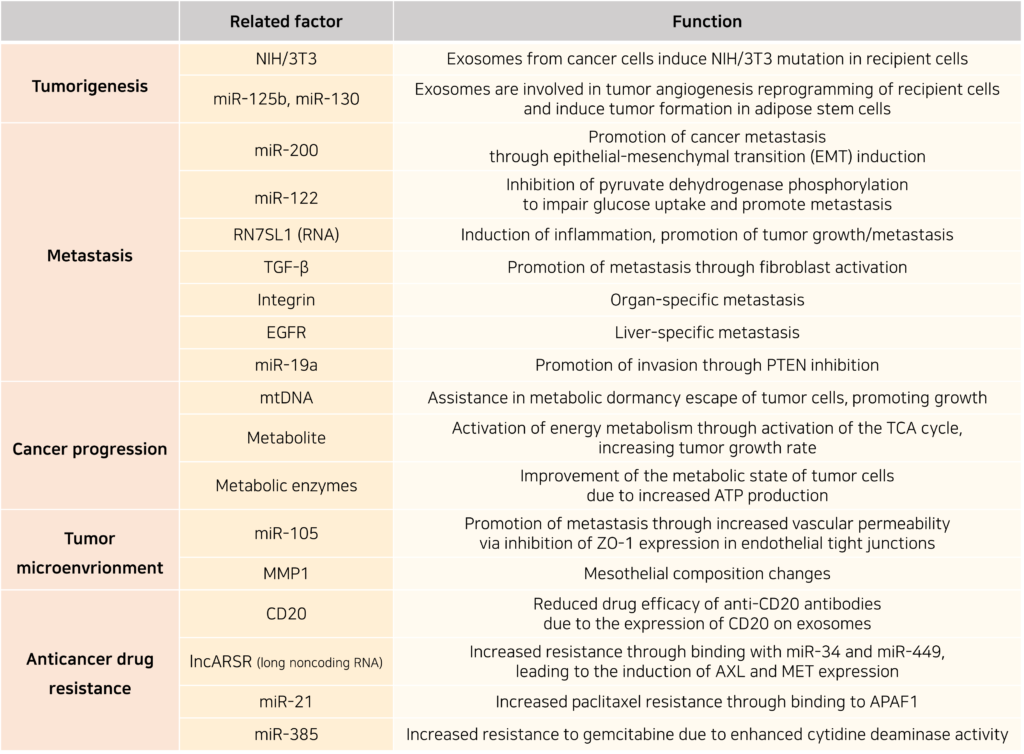
◎ Diagnostic applications of exosomes
Through our exploration of exosomes, we have confirmed their diverse roles in the immune system as well as in the onset and progression of various diseases. The fact that exosomes serve as essential components in numerous metabolic mechanisms and disease pathways suggests that they can be utilized to diagnose metabolic states and monitor disease progression.
Since exosomes are found in all bodily fluids and are secreted by all cell types, they present an attractive tool for monitoring disease status through minimally invasive liquid biopsies. Given that exosomes encompass both intracellular and extracellular molecules, they can serve as comprehensive indicators of various physiological conditions.
In practice, even a small amount of DNA extracted from exosomes can be sufficient to detect cancer-related mutations. Exosomal DNA typically covers a broader range than circulating free DNA (cfDNA), making it easier to identify mutations in genes such as KRAS and TP53.
In addition to DNA, miRNA also plays a crucial role in cancer diagnosis and prognosis assessment. miRNA profiles often differ significantly between cancerous and normal cells, making them useful for early cancer detection. For example, elevated levels of miR-21 in blood exosomes have been linked to brain tumors, pancreatic cancer, colorectal cancer, and liver cancer. Increased miR-21 levels in urine exosomes are associated with bladder and prostate cancer. Other cancer-related miRNAs include miR-155, miR-17-92, and miR-1246, which can serve as potential biomarkers for cancer development.
Exosomal proteins can also serve as diagnostic markers. For instance, the detection of GPC1-positive exosomes is strongly correlated with pancreatic, breast, and colorectal cancers. The relative abundance of phosphatidylserine (PtdSer), a lipid component of exosomes, is also a key indicator for early cancer diagnosis. By analyzing exosomal proteins, lipids, RNA, and miRNA together, it is expected that the specificity, sensitivity, and prognostic accuracy of cancer diagnosis will be significantly improved.
◎ Therapeutic applications of exosomes
As previously discussed, exosomes are emerging as a novel therapeutic alternative. In particular, exosomes derived from mesenchymal stem cells (MSCs) or epithelial cells have demonstrated excellent therapeutic potential when injected into mice, without inducing toxicity. As a result, treatment strategies utilizing these exosomes are currently under development.
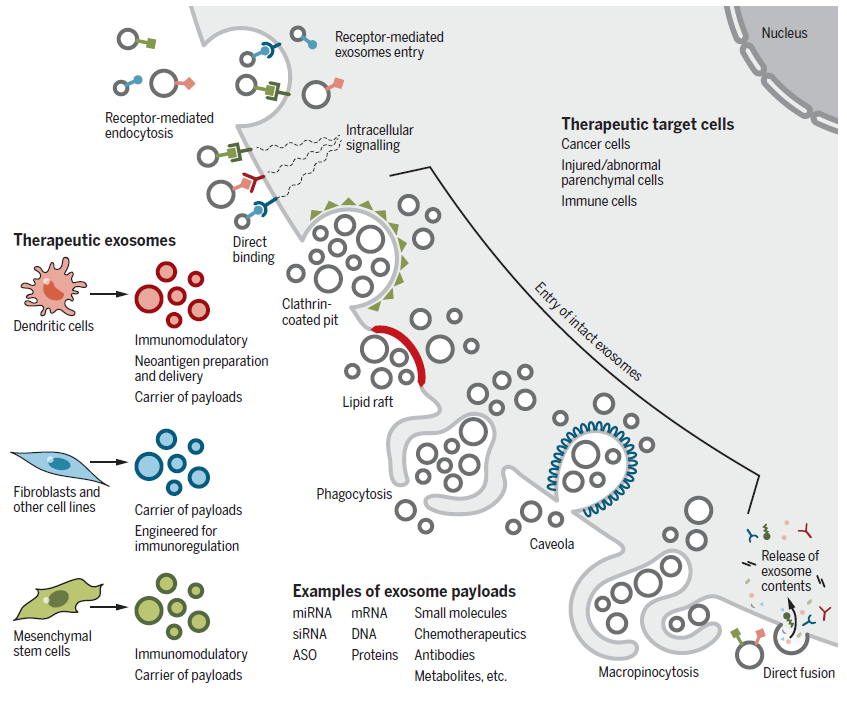
Exosomes have also gained recognition for their advantages as drug delivery vehicles, making this an area of ongoing research. Unlike liposomes, exosomes can efficiently penetrate cells when injected into the body. Additionally, they offer a breakthrough advantage in minimizing immune-related side effects caused by the therapeutic agents they carry. Furthermore, when delivering nucleic acids such as RNA, exosomes protect them from enzymatic degradation by ribonucleases in the bloodstream, thereby increasing their half-life and ensuring stable delivery to distant target sites.
Among exosomal cargo, miRNAs have been found to play a crucial role in metabolic regulation, leading to research on engineered exosomes for therapeutic applications. By engineering exosomes to carry specific miRNAs or siRNAs, they can be used to regulate target gene expression for disease treatment. For instance, miR-146b-loaded MSC exosomes have been shown to specifically regulate EGFR expression in brain tumors. Similarly, MSC exosomes carrying KrasG12D siRNA have been tested in various animal models for pancreatic cancer treatment. Moreover, to prevent the loss of exosomes in circulation, overexpression of CD47, which inhibits phagocytosis, was found to increase exosomal half-life.
The ability of exosomes to cross the blood-brain barrier (BBB) has opened new possibilities for treating previously untreatable brain-related diseases. In a study, intranasal administration of MSC-derived exosomes alleviated autistic-like behavior in mice. Additionally, intravenous administration of exosomes in mice with traumatic brain injury (TBI) demonstrated neuroprotective effects. In another study, dendritic cell-derived exosomes engineered to express rabies virus glycoprotein (RVG) and loaded with siRNA targeting BACE1 successfully inhibited BACE1 expression in the brain, highlighting their potential for Alzheimer’s disease treatment. Similarly, siRNA-loaded exosomes targeting α-synuclein reduced α-synuclein aggregates in the brain, alleviating pathological conditions. Exosomes have also been used as dopamine delivery carriers for Parkinson’s disease, demonstrating superior efficacy and reduced toxicity compared to direct dopamine injection therapy.
Another promising application of exosomes is targeting the tumor microenvironment (TME) as an emerging cancer treatment approach. As previously mentioned, dendritic cell-derived exosomes exhibit immunomodulatory effects by presenting antigens. A specific type of engineered exosome, known as “dexosomes”, is derived from IFN-γ-matured dendritic cells and has been shown to enhance NK cell-mediated cytotoxicity when loaded with the MART1 peptide.
Although extensive research has uncovered significant insights into the physiological and pathological roles of exosomes, further investigation is needed before they can be fully utilized for diagnostic and therapeutic purposes. While exosome-based therapies have demonstrated efficacy, the precise mechanisms through which they exert their effects remain unclear. Future studies must focus on identifying exosomal characteristics (e.g., heterogeneity, internal and external composition, and functional properties) to advance their therapeutic potential and unlock broader applications in medicine.
Reference : Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 Feb 7;367(6478):eaau6977.