The apoptotic capability of T cells has gained significant attention as a key component of the immune response against cancer. Ongoing research on T cells has led to the emergence of new strategies in cancer treatment, including immune checkpoint blockade, adoptive cellular therapy, and cancer vaccinology. In the upcoming series, we will delve into the principles and types of these immunotherapies.
The birth and evolution of immunotherapy for cancer treatment
The efforts to treat cancer by harnessing the immune response began in the 19th century. Wilhelm Busch and Friedrich Fehleisen were among the first to discover the dynamics between the immune system and cancer. They observed that tumors decreased simultaneously with bacterial skin infections.

Subsequently, William Coley, often regarded as the father of immunotherapy, demonstrated that this skin infection aided in the treatment of sarcoma. Building upon this, Coley conducted cancer treatment using bacterial extracts to stimulate the immune response. The substance, known as Coley’s toxin, exhibited effects across various cancer types. However, due to the unclear mechanism of action of Coley’s toxin and the emergence of radiation therapy and chemotherapy, it did not gain recognition as a standard cancer treatment.
In the 20th century, immunotherapy for cancer treatment regained attention. Numerous scientists demonstrated the hypothesis that the body’s immune system resists cancer, as seen in phenomena such as graft rejection reactions, through research on immune responses to tumor cells. As the significant impact of T cells on cancer became evident within the realm of immune cells, the scientific basis for immunotherapy was established.
Current immunotherapy approaches are emerging as innovative strategies to conquer cancer, aiming to elevate patient survival rates. Ongoing research on combination therapies and novel drug targets contributes to the steady expansion of the achievements in immunotherapy for cancer treatment.
In this series, we aim to explain three prominent strategies of immunotherapy for cancer treatment: immune checkpoint blockade, adoptive cellular therapy, and cancer vaccines. Additionally, we will introduce newly researched targets and approaches. Before delving into these strategies, today’s article will first explore T cells, the foundation of all these approaches.
The functions and lifecycle of T cells
At the core of adaptive immunity are two types of cells: T cells and B cells. B cells recognize circulating antigens in the body and induce an immune response by producing the antigen-specific antibodies. In contrast, T cells respond to antigens in the form of peptide fragments presented on the cell surface through Major Histocompatibility Complex (MHC), following their breakdown within antigen-presenting cells (APCs).
T cells can be broadly classified into two types based on the mechanism of their function, distinguished by the expression of CD4 or CD8 on the cell surface. CD4+ T cells recognize antigens presented on MHC II and regulate adaptive immune responses by producing immune-regulating cytokines. On the other hand, CD8+ T cells recognize antigens presented on MHC I, triggering cytotoxic responses to directly eliminate infected or cancerous cells.
The T cell receptor (TCR) they commonly possess is a protein complex on the cell surface that is clone-specific, playing a crucial role in the maturation and activation of T cells. Comprising various glycoproteins and signaling proteins, TCR undergoes rearrangements of these proteins. Through this rearrangement, clone-specific T cells are formed, growing into T cells capable of selective responses to external antigens after undergoing positive or negative thymic selection processes.
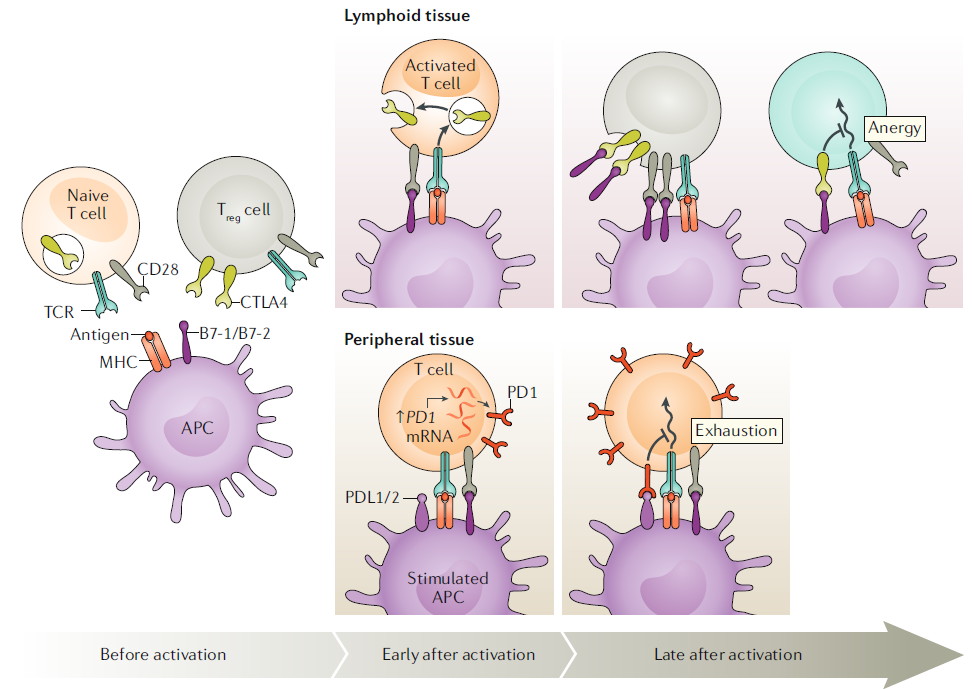
Antigen stimulation through TCR is essential for the function and proliferation of T cells. Phosphorylation necessary for intracellular signaling occurs through additional co-stimulation, which is initiated by surface receptors such as CD28. When signaling through both TCR and CD28 occurs simultaneously, T cells can undergo normal activation and proliferation. B7-1 and B7-2, which bind to the CD28 receptor, are expressed on antigen-presenting cells (APCs) and their expression increases when APCs are stimulated by antigen sensors such as Toll-like receptors (TLRs).
On the other hand, the expression of proteins like cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1), which bind to APC’s B7, is induced during the progression of the immune response. These proteins act as ‘immune checkpoints’ to prevent excessive activation of T cells. The signals transmitted through TCR lead to downstream signaling pathways through lymphocyte-specific protein kinase (LCK) within the cell via CD3. This process is normally inhibited by the surface protein CD45, but upon T cell stimulation, the inhibition is released, allowing the progression of downstream signaling.
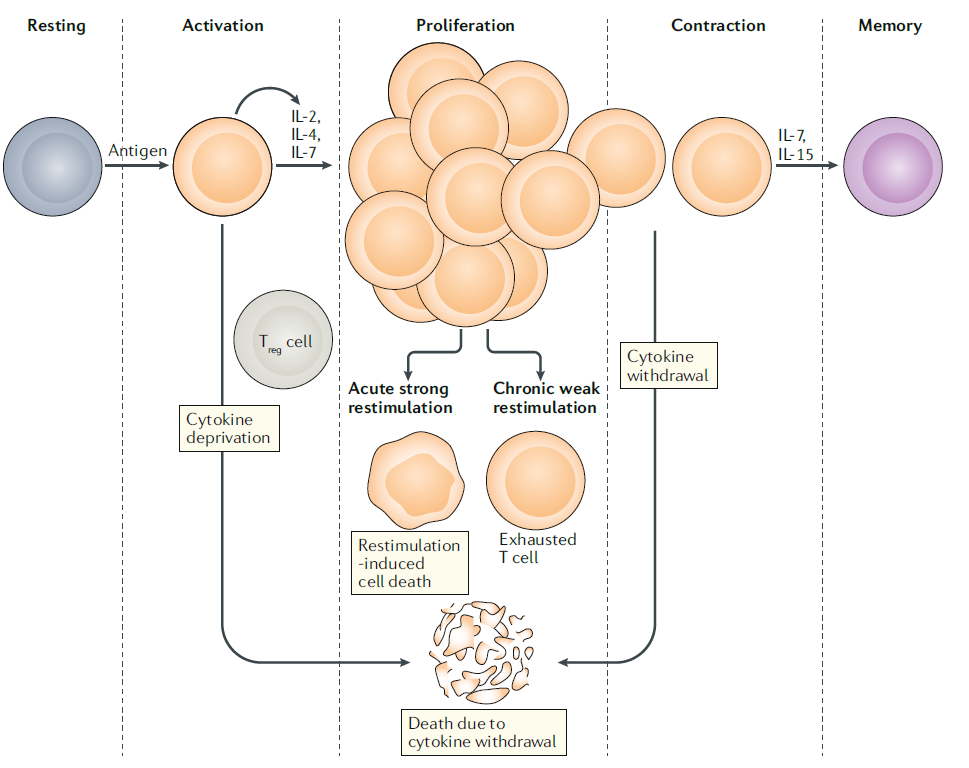
The processes following T cell activation can be classified into three categories. Firstly, the number of cells decreases due to the completion of the immune response or cell death caused by persistent overstimulation. Secondly, T cell dysfunction due to repeated low level stimulation can lead to the development of conditions we commonly know as chronic infections or tumor formation. Lastly, some of the activated T cells become memory T cells, participating in long-term immune memory. These memory T cells can trigger a more explosive immune response when exposed to the same antigen, serving as mediators for immune responses against antigens and cancer cells.
Thanks to the recent advancements in molecular biology, research on the characteristics and functions of T cells, especially those capable of infiltrating tumors, is actively progressing through technologies such as recent single-cell sequencing. Consequently, immunotherapy is continually evolving with the development of new targets and approaches, indicating that the conquest of cancer is not far off. In the upcoming article, we will delve into immune checkpoint inhibitors in detail.
Reference : Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020 Nov;20(11):651-668. doi: 10.1038/s41577-020-0306-5.
