In the previous text, we summarized the history of immune checkpoint inhibitors and the functions of T cells, which form the basis for these therapies. Now, let’s delve into the first of the three introduced immune checkpoint inhibitors: ‘Immune Checkpoint Blockade.’
Immune checkpoint inhibitors that prevent the activation of T cells play a role in preventing an excessive immune response. CTLA-4 and PD-1 are representative examples, and they act on specific areas and at specific times on T cells. These two molecules are complementary, maintaining self-tolerance while enabling selective responses to antigens and tumors, thereby protecting the body.
Biological function of CTLA4
CTLA-4 shares structural and biochemical similarities with CD28. However, one key difference lies in their expression patterns. While CD28 is constitutively expressed at high levels in conventional T cells, CTLA-4 is expressed at lower levels and is strongly induced after T cell activation. The extracellular domain of these two molecules exhibit high structural similarity, allowing them to bind to the same ligands. For instance, both CTLA-4 and CD28 can bind to B7-1 and B7-2, which are expressed on antigen-presenting cells (APCs). CTLA-4, however, has a stronger binding affinity for B7 proteins compared to CD28, and this difference influences the activation mechanism of T cells.
These two molecules possess opposing immune regulatory functions. CTLA-4, with its stronger binding to B7 proteins, competitively inhibits the activation and proliferation of T cells, acting in opposition to CD28. It also influences the signal transduction system of the T cell receptor (TCR). The fact that deficiency in the CTLA-4 gene leads to the onset of autoimmune diseases suggests that CTLA-4 acts as a factor that inhibits T cells and maintains self-tolerance. CTLA-4 achieves inhibition of CD28 signaling through direct interference, competitive action with co-stimulatory ligands, disruption of antigen binding, and recruitment of inhibitory factors. As a result, it regulates the activity of transcription factors, inducing a state of T cell anergy.
CTLA4 blockade in cancer
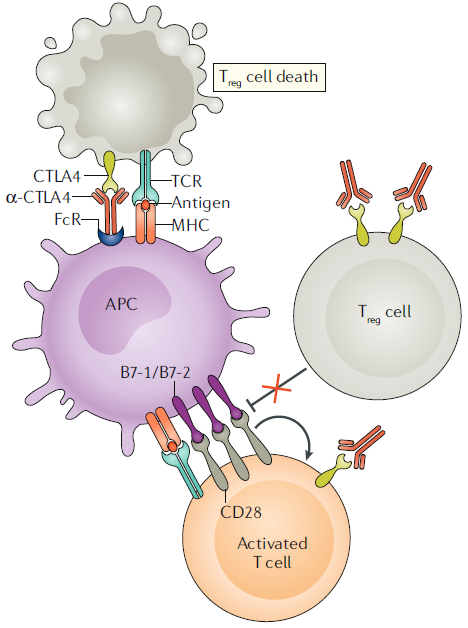
The discovery of CTLA-4’s role as a negative regulator of T cells has drawn attention to CTLA-4 blockade as a novel target for anticancer therapy utilizing T cell activation. Animal experiments have shown that the use of anti-CTLA-4 antibodies leads to the elimination of cancer cells and induces long-term immune memory. However, it has been observed that the effectiveness is limited, especially when dealing with larger tumors that face barriers within the tumor microenvironment. Despite these challenges, in 2011, the anti-CTLA-4 monoclonal antibody drug Ipilimumab received FDA approval for the treatment of melanoma, contributing to increased short-term and long-term survival rates for patients.
The mechanisms underlying tumor elimination through CTLA-4 involve various pathways, ultimately converging on the action of T cells. Inhibition of CTLA-4 enhances the anticancer response of effector T cells, leading to a reduction in regulatory T cells within the tumor. This alteration in the immune-suppressive tumor microenvironment is achieved through T cell activity. However, the precise anticancer mechanisms are not yet fully understood, and it is currently speculated that the ratio of effector T cells to regulatory T cells may be a critical factor.
Biological function of PD1/PDL1
PD-1, like CTLA-4, functions as an inhibitor of excessive immune responses. PD-1 is expressed on T cells after TCR stimulation and binds to PDL1/PDL2 expressed on APCs, and this binding is referred to as the ‘PD-1 axis.’ The PD-1 axis enables T cells to develop tolerance to self-antigens. Functionally similar to CTLA-4, the key difference is that PD-1 becomes active in peripheral tissues, unlike CTLA-4, which predominantly acts in lymphoid organs.
PD-1 blocks immune responses through inhibitory signal transduction within T cells. However, unlike CTLA-4, PD-1 is involved not only in the inhibitory effects on T cells but also in their sustained activation, proliferation, and, notably, the differentiation process of regulatory T cells. Generally, when PD-1 binds to ligands such as PDL1, it induces T cell exhaustion, regulating immune responses. Unfortunately, tumour cells can exploit this mechanism. Tumour cells may express PDL1 on their surface, similar to APCs, inducing T cell exhaustion, or create a tumor microenvironment conducive to tumor growth, evading immune surveillance.
PD1/PDL1 blockade in cancer

With the discovery of the functions of the PD-1 axis, research has been conducted to apply this knowledge to cancer immunotherapy. Blocking the PD-1 axis using monoclonal antibodies, for instance, has been observed to restore cytotoxicity of CD8+ T cells against cancer cells, leading to reduced tumor size or halting further growth in animal experiments. Additionally, PD-1 inhibition not only increases anticancer responses but also demonstrated the ability to prevent the spread of cancer cells through the bloodstream. Therefore, PD-1/PDL-1 blockade has become a therapeutic approach that can simultaneously inhibit tumor cell lysis and metastasis.
Based on these findings, monoclonal antibodies that block the PD-1 axis were developed. In 2014, pembrolizumab and nivolumab received FDA approval as the first immune checkpoint inhibitors for metastatic melanoma. These drugs significantly improved patient survival rates compared to previously used chemotherapy drugs, and better prognosis was observed with higher expression of PDL-1 in tumors. An intriguing point is that PD-1 inhibitors can be used for the treatment of various cancer types, unlike CTLA-4 inhibitors. While the exact reason is unclear, a plausible hypothesis suggests that if CTLA-4 is involved in a broader range of immune regulation mechanisms, PD-1 may be more tumor-specific.
Blocking PDL-1 through antibodies also exhibits anticancer effects. In 2016, atezolizumab received its first FDA approval for the treatment of urothelial carcinoma. Similar to PD-1, the therapeutic effects through PDL-1 blockade vary depending on the expression levels of PDL-1 in tumors.
Adverse effects of checkpoint blockade
Immune checkpoint inhibitors demonstrate potent immune-boosting effects, yet they can also lead to loss of immune tolerance to self-antigens. When immune checkpoint inhibitors are used, immune-related adverse events are observed in 15-90% of patients. The primary symptoms of these side effects include inflammation reactions in organs due to deficiencies in naïve T cells and excessive activation of memory T cells. Notably, drugs targeting the CTLA-4 pathway, unlike those targeting the PD-1 axis, have been associated with severe autoimmune complications, and symptom exacerbation has been reported with increased dosage. Adverse effects in anti-CTLA-4 therapy commonly manifest in the gastrointestinal tract and brain, while anti-PD-1 therapy may result in symptoms such as hypothyroidism, hepatotoxicity, and pneumonia.
However, immune checkpoint inhibitors exhibit much lower toxicity compared to traditional treatments like chemotherapy. Continuous research focuses on modifying antibodies or engineering drug delivery methods to improve side effects, making them still prominent as next-generation anticancer agents. Recent insights into the mechanisms behind the side effects caused by CTLA-4 inhibitors have opened possibilities for enhancing the stability and effectiveness of therapies. Moreover, ongoing studies related to biomarkers allow the prediction of potential toxicities in patients. For instance, high expression of CEACAM1 or CD177 suggests the likelihood of gastrointestinal-related side effects during treatment with ipilimumab. The advancements in pharmacogenomics further enable the identification of genes associated with drug toxicity, paving the way for predicting and preventing side effects when using immune checkpoint inhibitors.
Understanding the mechanisms by which cancer cells evade the body’s immune system and blocking them with immune checkpoint inhibitors allows the immune system to spontaneously treat tumors with less toxicity compared to conventional chemotherapy. In the case of solid tumors with larger sizes, overcoming the barrier known as the tumor microenvironment represents a groundbreaking approach to conquering various cancer types.
Reference : Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020 Nov;20(11):651-668. doi: 10.1038/s41577-020-0306-5.
