A flow cytometer is a precision instrument widely used in the pharmaceutical and biotechnology fields to analyze the physical and physiological characteristics of individual cells, such as cell size, internal complexity, and surface proteins. It primarily detects the properties of cells by analyzing light emitted from fluorescent dyes and is capable of examining a large number of individual particles in a short amount of time.
These advantages make flow cytometry applicable not only in evaluating the efficacy and in vivo effects of therapeutic candidates during drug development, but also during process design. In particular, it can be utilized in the development and quality assessment of biologics such as cell-based products. By characterizing the product and ensuring process consistency, flow cytometry helps validate the stability of separation and purification processes.
Over the next two segments, we will explore the basic components, working principles, and analytical applications of flow cytometry. In this first part, we will focus on the key components and operational principles of the flow cytometer.
Principle of flow cytometer
Flow cytometers are generally categorized into two types: instruments designed solely for analysis, and those capable of both analysis and particle sorting based on the results. Instruments commonly referred to as FACS (Fluorescence-Activated Cell Sorters) fall into the latter category, incorporating sorting functions as part of the flow cytometer system.
A flow cytometer typically consists of four main components: the fluidics system, optical system, electronics, and computer. The fluidics system transports particles suspended in the sample fluid through a stream, guiding them toward the laser interrogation point. As the laser light hits each particle, it scatters or induces fluorescence emission. This light is detected by sensors and converted into electrical signals. These signals are then digitized, processed by a computer, and finally presented as data interpretable by the user.
While flow cytometry can analyze whole cells as individual particles, it also enables more detailed investigations of specific cellular components such as the membrane, cytoplasm, and nucleus. Beyond proteins, it can be used to identify and quantify cellular organelles, nucleic acids, cytokines, and hormones. With these capabilities, flow cytometry allows for the analysis of cell cycle stages, cell division, calcium flux, membrane potential, and other dynamic cellular events.
Components of a flow cytometer
1) Fluidics system
The fluidics system serves a physical role in allowing cells to travel through the instrument. Key components of the fluidics system include the sheath fluid and the pressurized airline. The sheath fluid, which can be considered a dilution buffer similar to PBS, transports the cells into the fluid chamber located adjacent to the pressurized airline. The pressurized airline applies pressure to drive the sample into the fluid chamber, maintaining an appropriate pressure differential. This ensures that the sample flows through the center of the stream, while the sheath fluid surrounds it, preserving a focused and stable flow for accurate analysis.
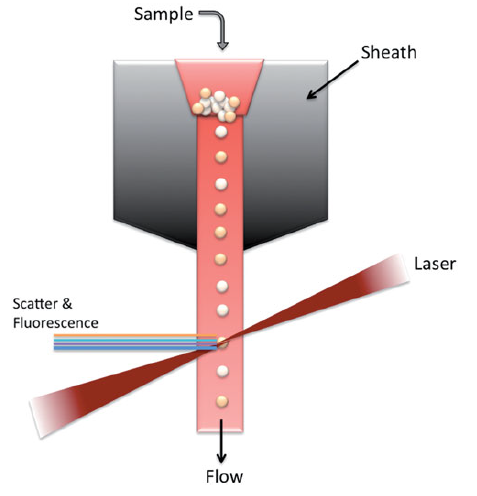
The hydraulic pressure of the sample is always kept higher than that of the sheath fluid to ensure that the cells flow in a single-file line. The sample flow rate is adjusted according to the specific analytical purpose. For instance, a higher flow rate is selected for high-throughput analyses such as immunotyping, whereas a lower flow rate is used in assays requiring high resolution, such as DNA content analysis, to improve accuracy.
Choosing the optimal fluid composition is also a critical factor for precise particle analysis. In addition, operators must constantly monitor the system to ensure there are no bubbles or residues in the fluid and that appropriate pressure is maintained, in order to minimize potential analytical errors.
2) Optical system
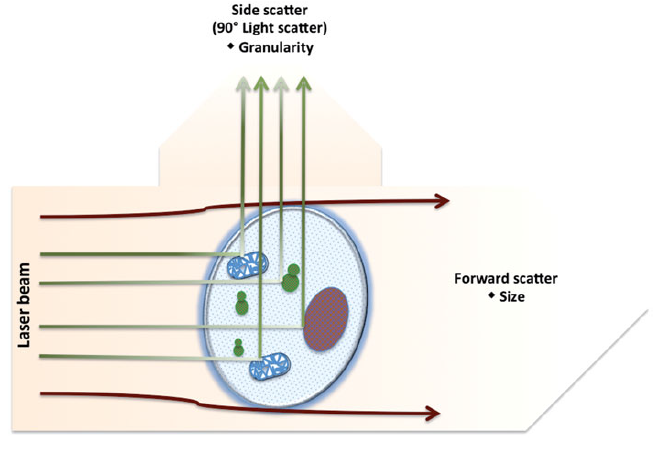
The optical system consists of lasers, lenses, and photon detectors. Lenses are responsible for shaping and focusing the laser beam. When the laser passes through the lens and strikes the cell, it excites electrons to higher energy orbits, leading to the emission of light. Photons are generated as these excited electrons return to lower energy levels. The emitted light then hits the cell and becomes refracted and scattered.
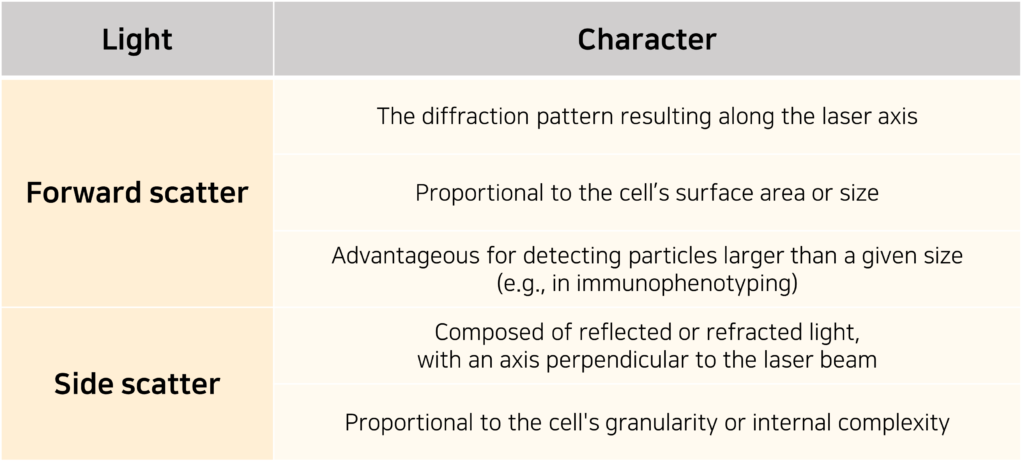
Factors that can influence the scattering of light include the cell membrane, nucleus, internal structural complexity, overall cell shape, and surface features. The scattered light is typically categorized into two types, as illustrated in the following table or diagram.
Depending on the type of fluorescent dye being used, various laser configurations can be applied. For example, an argon laser (488 nm) is commonly used with dyes such as FITC or those found in phytoplankton that emit light at higher wavelengths. Most flow cytometers are also equipped with additional lasers such as UV lasers (300–400 nm) or red diode lasers (630 nm).
The optical detection system consists of multiple lenses, optical mirrors, and filters. Lenses are used to collect light, and the reflected light from the mirrors passes through filters that selectively transmit light of specific wavelengths. The specificity of a detector for a particular fluorescent signal is influenced by whether appropriate filters are installed.
There are three main types of filters: band-pass filters, which allow only light within a specific wavelength range to reach the detector; short-pass filters, which transmit wavelengths equal to or shorter than a defined cutoff; and long-pass filters, which transmit wavelengths equal to or longer than a certain threshold.
3) Analysis system
Lasers that pass through the fluid are converted into voltage signals by optical detectors. Of the two main types of detectors, photomultiplier tubes (PMTs) are more sensitive than photodiodes (PDs) and more efficiently convert light into photon signals. Therefore, PDs are typically used to detect strong light signals such as those from forward scatter (FSC), while PMTs are used to detect weaker signals such as those from side scatter (SSC) or fluorescence.
The optical signals collected by PMTs and PDs generate current signals proportional to the number of electrons, which are then converted into voltage pulses through amplifiers. These analog current signals are processed by two types of amplifiers—linear and logarithmic—and subsequently converted into digital signals via a computer. When visualized as plots or histograms, these digital signals become the flow cytometry data we commonly encounter.
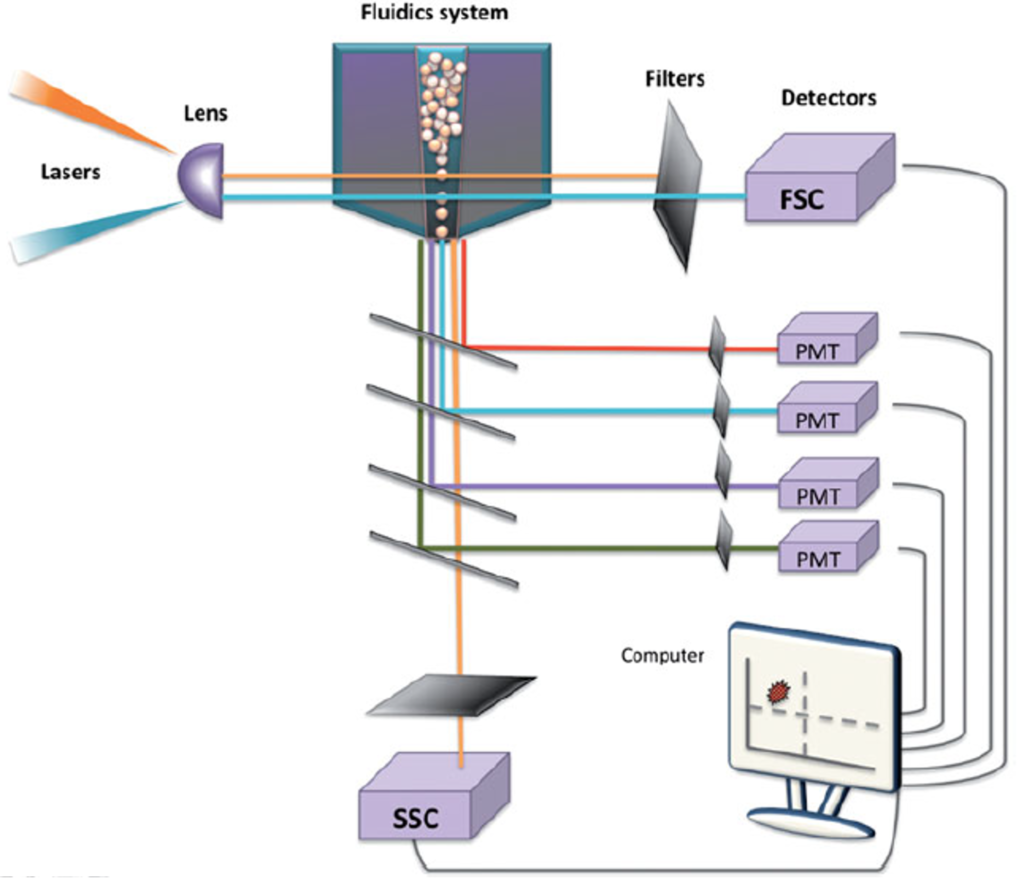
As seen from its components, a flow cytometer is an analytical instrument that utilizes light refraction and scattering. Even without any staining, the lasers inherently present in the flow cytometer can analyze forward scatter (FSC) and side scatter (SSC) to reveal various cellular characteristics. However, by using different types of fluorescent dyes, much more detailed and finer information can be collected and examined.
In the following section, we will further discuss the principles of cell analysis using fluorescent dyes, the methods for analyzing the resulting data, and the experiments that can be performed using a flow cytometer.
Reference : Adan A, Alizada G, Kiraz Y, Baran Y, Nalbant A. Flow cytometry: basic principles and applications. Crit Rev Biotechnol. 2017 Mar;37(2):163-176. doi: 10.3109/07388551.2015.1128876.