To ensure that a raw material with therapeutic effects can be processed into a drug and prescribed for actual treatment, its safety must be guaranteed. This is why the purity of pharmaceutical formulations must be ensured. If unintended substances are mixed into the formulation or if unidentified substances are combined due to incomplete component analysis, unpredictable side effects may occur in the body.
Therefore, to produce safe and high-purity pharmaceuticals, manufacturing processes are designed to include various purification techniques. During the design process, suitable purification methods are selected based on the chemical and physical properties of the target drug. Chromatography is the most commonly used purification technique in this process.
This article introduces the most commonly used chromatography techniques and examines the basic conditions for conducting purification using each method.
1. Affinity chromatography (AC)
In affinity chromatography, a resin with specific ligands attached is used to separate proteins through reversible interactions between the ligands and the target protein. This technique is suitable for use in early or intermediate purification stages and can be applied whenever a ligand that binds to the target protein is available. Affinity chromatography offers high selectivity, enabling the purification of high-purity proteins, and generally yields high recovery rates.
In a typical two-step purification process, affinity chromatography is used in the first stage (capture step) to isolate the target protein. In the second purification stage (polishing step), chromatography techniques are used to remove remaining impurities.
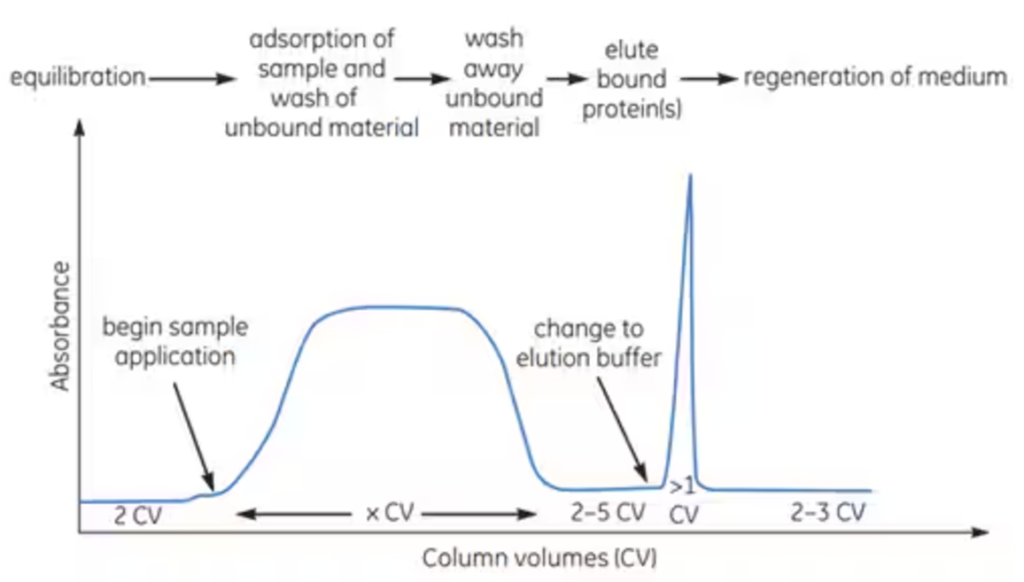
The target protein binds specifically and reversibly to the ligand. The sample undergoes purification under conditions that facilitate the interaction between the protein and the ligand. Unbound substances are washed away, and proteins bound to the ligand can be eluted by altering the conditions to favor separation. For specific elution, competitive ligands are employed, while nonspecific elution can be achieved by adjusting pH, ionic strength, or polarity conditions.
While bound to the resin, the sample is concentrated, and the target protein is recovered in a highly pure and concentrated form. The overall chromatogram of affinity chromatography appears as follows.
2. Ion exchange chromatography (IEX)
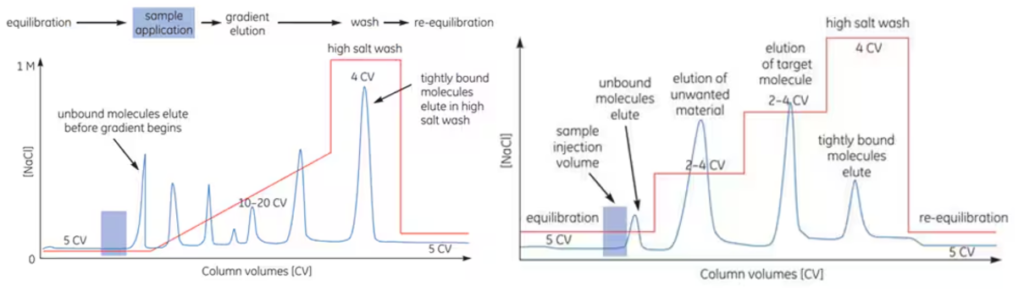
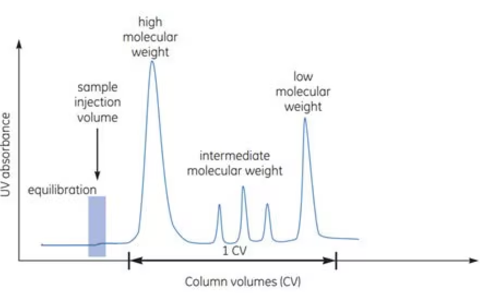
Ion exchange chromatography is a technique that separates proteins based on differences in their surface charges. It allows for high-resolution protein separation, making it suitable for processing complex samples with diverse mixtures. This chromatography method performs separation through reversible interactions between charged proteins and oppositely charged packing materials in the column. Proteins bind to the column immediately upon entry, and conditions can be adjusted to separate and elute different proteins. Elution is achieved by increasing the salt concentration or adjusting the pH. When controlling salt concentration, a stepwise or continuous linear gradient approach can be used. The most common method involves adjusting the sodium chloride concentration linearly for elution as shown in the figure below. In ion exchange chromatography, as with other techniques, the target protein is purified and concentrated to a high degree of purity and yield.
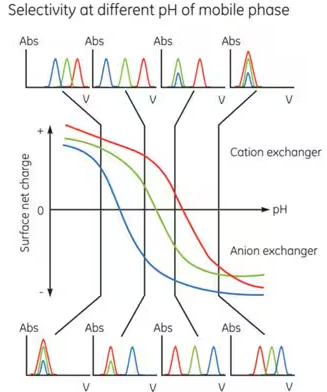
The actual charge on a protein’s surface varies depending on the pH of the surrounding solvent. Generally, proteins in a solvent with a pH higher than their isoelectric point will bind to an anion exchange resin, while proteins in a solvent with a pH lower than their isoelectric point will bind to a cation exchange resin. However, it is important to note that binding depends on the charge, and the surface charge is sufficient to bind on either side of the isoelectric point. Typically, ion exchange chromatography is used to separate the target molecule through binding, but it can also be used to bind and separate impurities when needed. Ion exchange can be repeated under different pH conditions to separate proteins with different properties (Figure 3).
3. Hydrophobic interaction chromatography (HIC)
Hydrophobic Interaction Chromatography (HIC) separates substances by utilizing differences in hydrophobicity among proteins. This technique is frequently employed during the concentration or intermediate purification stages of the purification process. Separation occurs through reversible interactions between the hydrophobic surfaces of the chromatography resin and the proteins, which are enhanced in high ionic strength solutions. Therefore, HIC is particularly suitable as a subsequent step after protein precipitation using ammonium sulfate or ion exchange chromatography with high salt concentrations.
Samples in high ionic strength solutions bind to the packing material upon contact. After binding occurs, conditions are adjusted to enable the sequential elution of the bound substances. Unlike ion exchange chromatography, HIC employs elution by decreasing the salt concentration (Figure 4). Similarly, a stepwise adjustment of salt concentration or a continuous linear gradient can be used. In most cases, elution is performed by gradually reducing the concentration of ammonium sulfate. Through this process, the target protein is concentrated and purified into a high-purity solution.
Additionally, other elution methods include reducing the polarity of the elution buffer (e.g., increasing the concentration of ethylene glycol), adding substances that disrupt hydrogen bonding (e.g., urea, guanidine hydrochloride), using surfactants, or adjusting pH or temperature.
4. Size exclusion chromatography (SEC)
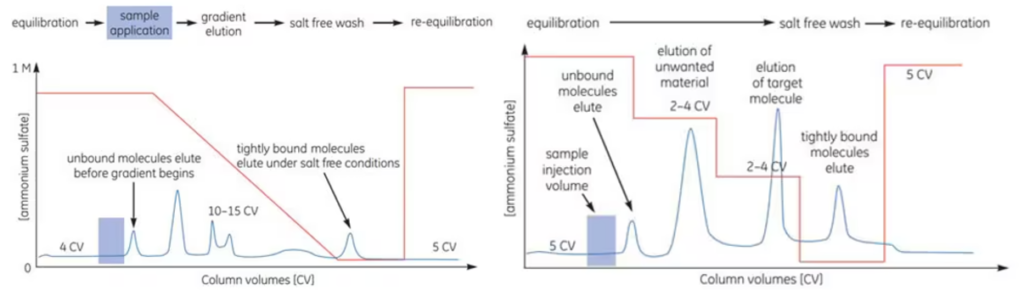
Size Exclusion Chromatography (SEC) is a technique used to separate proteins based on their size and shape. This chromatography method is commonly employed in the final stage of the purification process, particularly when the sample volume is small, to remove impurities. Since sample volume significantly affects the resolution and flow rate of the chromatography process, careful consideration of this factor is essential.
In SEC, an isocratic method is used for elution, where a single solvent is applied without any adjustment in concentration. The solvent conditions can be selected based on the type and characteristics of the sample, as well as subsequent purification, analysis, or storage steps. However, because the solvent plays a critical role in determining the resolution of SEC, it must be carefully selected thorough characterization.
As a result, the target protein is purified and concentrated in the selected solvent during this process.
5. Reversed phase chromatography (RPC)
When proteins are introduced into the column and come into contact with the packing material, they bind immediately. Similar to other previous techniques, separation is achieved by altering the binding conditions according to the specific properties of each molecule.
Reverse Phase Chromatography (RPC) is a technique that separates proteins and peptides based on differences in hydrophobicity, utilizing the reversible interactions between each molecule and the packing material. When proteins are applied into the column and come into contact with the packing material, they bind immediately. As with other techniques, separation is achieved by adjusting the binding conditions according to the specific properties of each molecule.
Due to the characteristics of the packing material used in RPC, the binding is typically very strong. The strength of this binding can be controlled by the use of organic solvents or other additives. For elution, the concentration of the organic solvent is gradually increased, with acetonitrile being a commonly used solvent for this purpose.
Through the processes of binding to and eluting from the packing material, the sample is both purified and concentrated, resulting in a chromatogram as shown below.
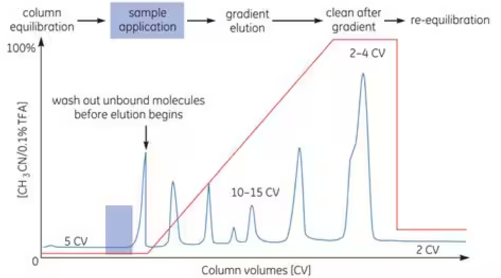
Reverse Phase Chromatography is primarily used in the final purification step to remove oligonucleotides or peptides and is also frequently used for analytical purposes, such as peptide mapping. However, when using reverse phase chromatography to purify proteins, it is not recommended for cases where the target protein requires activity restoration or tertiary structure recovery, as contact with organic solvents often leads to protein denaturation.
Chromatography techniques are methods used to separate complex mixtures into individual components based on their properties. As described above, these techniques are suitable not only for drug purification but also for use in analytical processes (e.g., HPLC, GC). While this text provides a brief overview of various chromatography techniques, designing a purification process using these methods allows for precise process design due to the countless variables that can be controlled. Selecting the appropriate chromatography technique based on the characteristics of the substance to be separated or analyzed, and controlling or adjusting the relevant variables, is a key element in producing high-purity and safe pharmaceutical products.
Reference : Principles and Standard Conditions for Different Purification Techniques (Merck), https://www.sigmaaldrich.com/KR/ko/technical-documents/technical-article/protein-biology/protein-purification/principles-and-conditions-for-purification-techniques